Solved The Copper Zinc Phase Diagram Is Shown You Would 47 Off
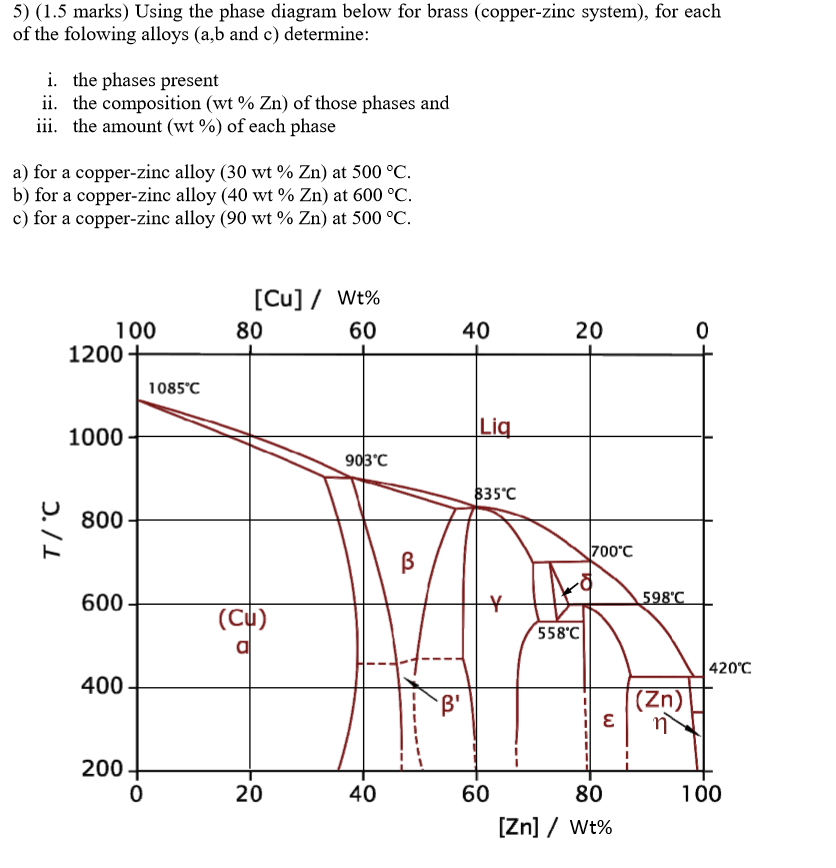
Solved The Phase Diagram For The Copper Zinc System Is 49 Off The copper zinc phase diagram is given. for this alloy to find: (a) the phases present (b) the composition of each phase (c) …. 4. the copper zinc phase diagram is shown below. you would consult this diagram to learn about phases in brass alloys. consider an alloy of 60% copper by weight and 40% zinc in equilibrium at a temperature of 700 °c. A region of the copper zinc phase diagram that has been enlarged to show eutectoid and peritectic invariant points , labeled e (5600 c, 74 wt% zn) and p (598 0 c, 78.6 wt% zn), respectively. figure by mit ocw. note that each single phase field is separated from other single phase fields by a two phase field.

Solved Figure Shows The Phase Diagram For The Copper Zinc 45 Off The cu zn phase diagram is a graphical representation of the different phases and their compositions that form when copper and zinc are combined. it provides valuable information about the phase transformations and solubility limits of these two metals at different temperatures. the phase diagram consists of a composition axis (expressed in. This diagram helps to explain the solidification behavior, phase transformations, and mechanical properties of copper zinc alloys. the phase diagram consists of a composition axis and a temperature axis. the composition axis represents the ratio of copper to zinc in the alloy, ranging from pure copper (100% copper) to pure zinc (100% zinc). 9.34 consider the hypothetical eutectic phase diagram for metals a and b, which is similar to that for the lead tin system, figure 9.8. assume that (1) α and β phases exist at the a and b extremities of the phase diagram, respectively; (2) the eutectic composition is 47 wt% b 53 wt% a; and (3) the composition of the β phase at the eutectic. Copper tin alloys: cu sn alloys are sometimes called bronzes, although this includes other kinds of copper alloys (e.g. with silicon and aluminium). the peritectic reaction (see diagram above) is an important example of a microstructural transformation. sn – 21wt.%cu exhibits this transformation from a solid phase and a liquid phase to a.

Comments are closed.