Solved Copper 64 Can Decay By Any Of The Three Beta Decay Processes
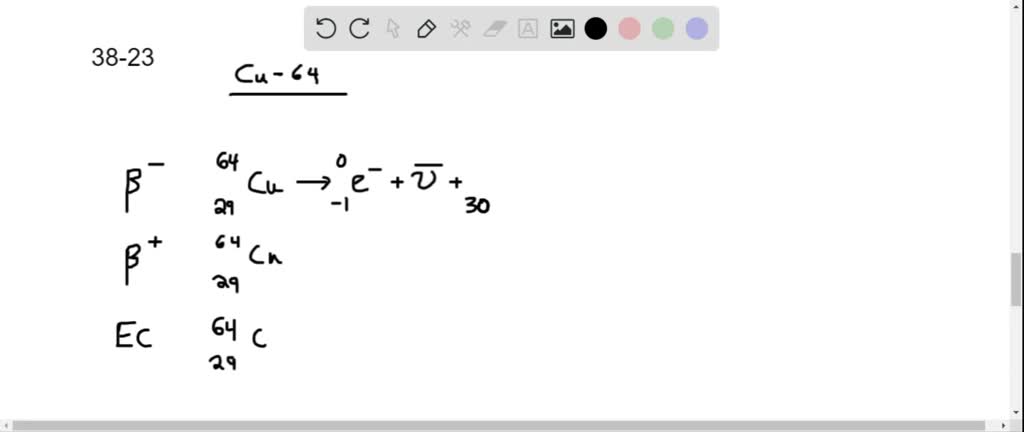
Solved Copper 64 Can Decay By Any Of The Three Beta Decay Processes Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer. question: copper 64 can decay by any of the three beta decay processes. write the equation for each decay. copper 64 can decay by any of the three beta decay processes. write the equation for each decay. In the beta minus decay, cu 64 changes a neutron into a proton and emits an electron (beta particle) and an antineutrino. we write this equation as: 29 64 cu → 30 64 zn e − v ¯ e. where 30 64 zn is the zinc atom obtained after decay, e − is the beta particle (electron), and v ¯ e is an electron antineutrino. 02.
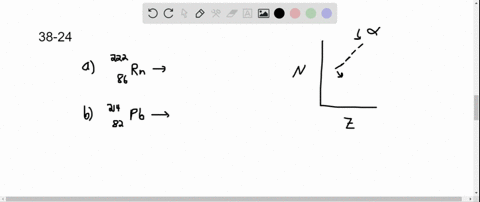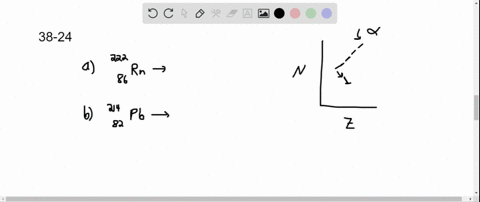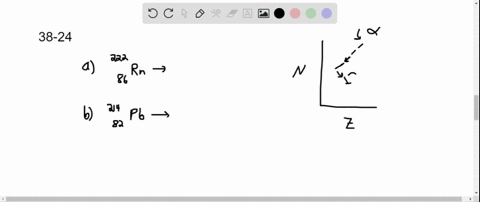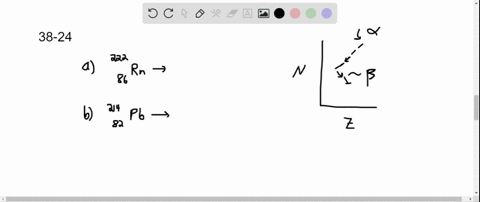
Solved Copper 64 Can Decay By Any Of The Three Beta Decay Processes In the first decay process, copper 64 transforms into zinc 64 by emitting a beta minus particle and an electron antineutrino the equation is balanced in terms of atomic and mass numbers. 2. the second decay involves the conversion of copper 64 into nickel 64 by emitting a beta plus particle and an electron neutrino this equation follows conservation laws for charge, lepton number, and mass number. The equation for beta minus decay of copper 64 is: cu 64 → ni 64 e ν¯ step 2 3 2. beta plus decay: in this process, a proton in the nucleus of copper 64 is converted into a neutron, and a positron and a neutrino are emitted. the equation for beta plus decay of copper 64 is: cu 64 → zn 64 e ν answer 3. Copper 64 (64 cu) is a positron and beta emitting isotope of copper, with applications for molecular radiotherapy and positron emission tomography. its unusually long half life (12.7 hours) for a positron emitting isotope makes it increasingly useful when attached to various ligands , for pet and pet ct scanning. Step 1 3 1. beta minus decay (β decay): in this process, a neutron is converted into a proton, and an electron (beta particle) and an electron antineutrino are emitted. the equation for this decay is: cu 64 (29 protons, 35 neutrons) → ni 64 (28 protons, 36 neutrons) e (electron) νe (electron antineutrino) step 2 3 2.

Solved Copper 64 Can Decay By Any Of The Three Chegg Copper 64 (64 cu) is a positron and beta emitting isotope of copper, with applications for molecular radiotherapy and positron emission tomography. its unusually long half life (12.7 hours) for a positron emitting isotope makes it increasingly useful when attached to various ligands , for pet and pet ct scanning. Step 1 3 1. beta minus decay (β decay): in this process, a neutron is converted into a proton, and an electron (beta particle) and an electron antineutrino are emitted. the equation for this decay is: cu 64 (29 protons, 35 neutrons) → ni 64 (28 protons, 36 neutrons) e (electron) νe (electron antineutrino) step 2 3 2. Question 23. it tells us that copper 64 can undergo any of the three, um, being educate beta decay radioactive processes. and you're gonna find out what the reactions would be in this scenario, the decayed equations being this scenario. so you think of the three, um, beta decay processes. As an example, 64 cu, a very important radioisotope frequently used in positron emission tomography [1,3,[9] [10] [11][12][13][14] and considered for use in radiotherapy [10,13,14], is produced in.
Copper 64 Isotopic Data And Properties Question 23. it tells us that copper 64 can undergo any of the three, um, being educate beta decay radioactive processes. and you're gonna find out what the reactions would be in this scenario, the decayed equations being this scenario. so you think of the three, um, beta decay processes. As an example, 64 cu, a very important radioisotope frequently used in positron emission tomography [1,3,[9] [10] [11][12][13][14] and considered for use in radiotherapy [10,13,14], is produced in.

Comments are closed.