Solved Consider The Following Decay Series A T1 2 4 5 Chegg

Solved Consider The Following Decay Series A T1 2 4 Consider the following decay series: a(t1 2=4.5 s) b(t1 2=22.0 days ) c if we start with 1.00×106 atoms of a, how many atoms of a,b, and c are there after 30.0 days? this problem has been solved!. Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer. question: consider the following decay series:a (t1 2 = 4.5 s) right arrow b (t 1 2 = 22.0 days) right arrow c if we start with 1.00 x 10^6 atoms of a, how many atoms of a, b, and c are there after 30.0 days.

Solved Consider The Following Decay Series A T1 2 4 , o r nlog2 =()1 6.7 , w hic give s 4.0 thus the time is t =nt 1 2 =()4.06 ()5730 yr = 2.3×104 yr. 4. an amateur archeologist finds a bone that she believes to be from a dinosaur and she sends a chip off to a laboratory for 14c dating. the lab finds that the chip contains 5g of carbon and has an activity of 0.5 bq. N = n0 2n. if the decay constant (λ) is large, the half life is small, and vice versa. to determine the relationship between these quantities, note that when t = t1 2, then n = n0 2. thus, equation 10.4.4 can be rewritten as. n0 2 = n0e − λt1 2. dividing both sides by n0 and taking the natural logarithm yields. Video answer: we want to figure out how much of the substances is left after this decay series, we're told here that we want to look at nuclear chemistry. we're told that we're going to go through a total of 30 days, starting with one mole sample. Radioactive dating. radioactive dating is a technique that uses naturally occurring radioactivity to determine the age of a material, such as a rock or an ancient artifact. . the basic approach is to estimate the original number of nuclei in a material and the present number of nuclei in the material (after decay), and then use the known value of the decay constant λ λ and equation 10.10 to.
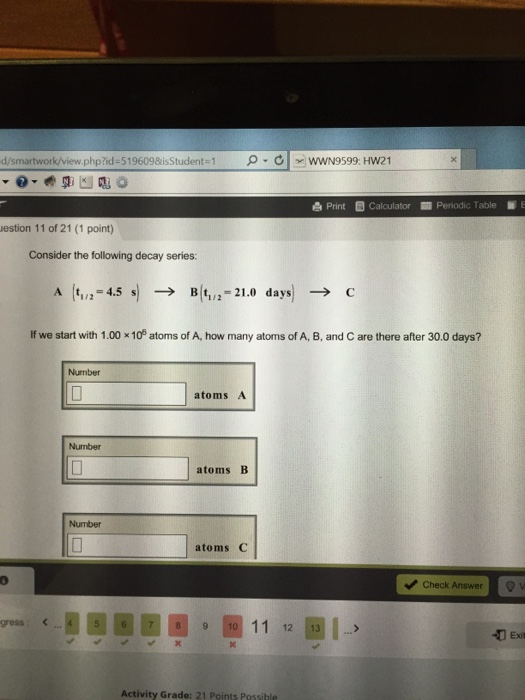
Solved Consider The Following Decay Series A T 1 2 4 Video answer: we want to figure out how much of the substances is left after this decay series, we're told here that we want to look at nuclear chemistry. we're told that we're going to go through a total of 30 days, starting with one mole sample. Radioactive dating. radioactive dating is a technique that uses naturally occurring radioactivity to determine the age of a material, such as a rock or an ancient artifact. . the basic approach is to estimate the original number of nuclei in a material and the present number of nuclei in the material (after decay), and then use the known value of the decay constant λ λ and equation 10.10 to. Summary. the half life of a reaction is the time required for the reactant concentration to decrease to one half its initial value. the half life of a first order reaction is a constant that is related to the rate constant for the reaction: t 1 2 = 0.693 k. radioactive decay reactions are first order reactions. Types of radioactive decay. ernest rutherford’s experiments involving the interaction of radiation with a magnetic or electric field (figure 21.6) helped him determine that one type of radiation consisted of positively charged and relatively massive α particles; a second type was made up of negatively charged and much less massive β particles; and a third was uncharged electromagnetic.
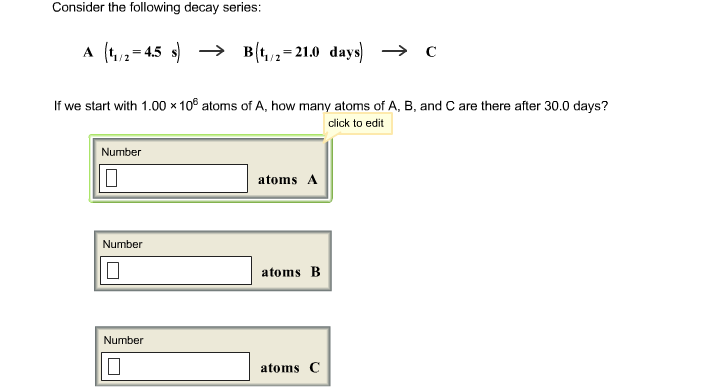
Consider The Following Decay Series A T1 2 4 5 S Summary. the half life of a reaction is the time required for the reactant concentration to decrease to one half its initial value. the half life of a first order reaction is a constant that is related to the rate constant for the reaction: t 1 2 = 0.693 k. radioactive decay reactions are first order reactions. Types of radioactive decay. ernest rutherford’s experiments involving the interaction of radiation with a magnetic or electric field (figure 21.6) helped him determine that one type of radiation consisted of positively charged and relatively massive α particles; a second type was made up of negatively charged and much less massive β particles; and a third was uncharged electromagnetic.

Solved Consider The Following Decay Series A T1 2 4

Comments are closed.