Reading Heating And Cooling Curves

Heating And Cooling Curves Read Chemistry Ck 12 Foundation The experiment described above can be summarized in a graph called a heating curve (figure below). figure 13.18.1 13.18. 1: in the heating curve of water, the temperature is shown as heat is continually added. changes of state occur during plateaus, because the temperature is constant. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2 . the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would.

Heating And Cooling Curves For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at. 2861°c. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. This is a chemistry tutorial video that goes through how to read and interpret heating curves or cooling curves. there are several examples of different ques. The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2. the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific.
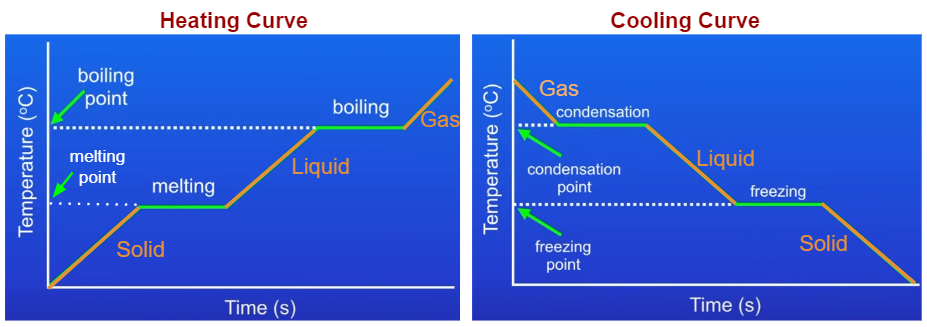
Heating And Cooling Graphs Examples Solutions Videos Notes The heating curve for carbon dioxide would have only one plateau, at the sublimation temperature of co 2. the entire experiment could be run in reverse. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. the water could then be cooled to 0°c, at which point continued cooling would. Boil water. heat steam from 100 °c to 120 °c. the heat needed to change the temperature of a given substance (with no change in phase) is: q = m × c × Δ t (see previous chapter on thermochemistry). the heat needed to induce a given change in phase is given by q = n × Δ h. using these equations with the appropriate values for specific. For example, this is the heating curve for iron, a metal that melts at 1538°c and boils at 2861°c. cooling curves. heating curves show how the temperature changes as a substance is heated up. cooling curves are the opposite. they show how the temperature changes as a substance is cooled down. just like heating curves, cooling curves have. 11.12 : heating and cooling curves. when a substance—isolated from its environment—is subjected to heat changes, corresponding changes in temperature and phase of the substance is observed; this is graphically represented by heating and cooling curves. for instance, the addition of heat raises the temperature of a solid; the amount of heat.

Comments are closed.