Isothermal Cooling Diagram
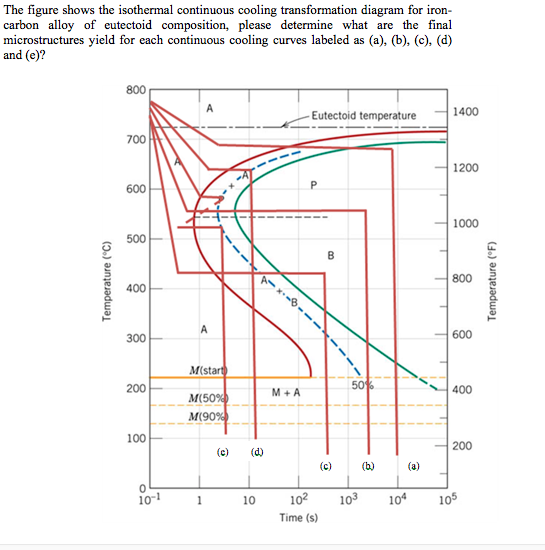
Solved The Figure Shows The Isothermal Continuous Cooling Chegg After cooling and holding at 700°c for 104 s, approximately 50% of the specimen has transformed to coarse pearlite. upon cooling to room temperature, the remaining 50% transforms to martensite. hence, the final microstructure consists of about 50% coarse pearlite and 50% martensite. (b) reheat the specimen in part (a) to 700°c (1290°f) for 20 h. T (time) t (temperature) t (transformation) diagram is a plot of temperature versus the logarithm of time for a steel alloy of definite composition. it is used to determine when transformations begin and end for an isothermal (constant temperature) heat treatment of a previously austenitized alloy. when austenite is cooled slowly to a.

Schematic Diagrams Illustrating Isothermal Curves It Critical Isothermal transformation diagram. Isothermal transformation and cooling transformation diagrams, american society for metals, 1997, p. 28.) effect of cooling history in fe c system 400 500 600 700 0 % p e a r l i t e 1 0 5 austenite (stable) t e (727°c) austenite (unstable) pearlite t(°c) 110102 103 104 105 time (s) γ γ γ γ γ γ. Salt bath i (fig. 1) is maintained at austenetising temperature (780 ̊c for eutectoid steel). salt bath ii (fig. 2) is maintained at specified temperature at which transformation is to be determined (below ae1), typically 700 250°c for eutectoid steel. bath iii which is a cold water bath is maintained at room temperature. For Δu Δ u we can substitute the expression for internal energy in equation 4.5.3 4.5.3 we obtained from the first law of thermodynamics. this gives us. Δh = q − pΔv pΔv = q (4.5.6) (4.5.6) Δ h = q − p Δ v p Δ v = q. so at constant pressure, the enthalpy change during a reaction is simply equal to the heat entering the system.

Difference Between Isothermal And Continuous Cooling Transformation Salt bath i (fig. 1) is maintained at austenetising temperature (780 ̊c for eutectoid steel). salt bath ii (fig. 2) is maintained at specified temperature at which transformation is to be determined (below ae1), typically 700 250°c for eutectoid steel. bath iii which is a cold water bath is maintained at room temperature. For Δu Δ u we can substitute the expression for internal energy in equation 4.5.3 4.5.3 we obtained from the first law of thermodynamics. this gives us. Δh = q − pΔv pΔv = q (4.5.6) (4.5.6) Δ h = q − p Δ v p Δ v = q. so at constant pressure, the enthalpy change during a reaction is simply equal to the heat entering the system. Effect of cooling history in fe c system. eutectoid composition, co = 0.76 wt% c. begin at t > 727°c. rapidly cool to 625°c and hold isothermally. adapted from fig. 10.14,callister 7e. (fig. 10.14 adapted from. h. boyer (ed.) atlas of isothermal transformation and cooling transformation diagrams, american society for metals, 1997, p. 28.). An isothermal process is a type of thermodynamic process in which the temperature t of a system remains constant: Δt = 0. this typically occurs when a system is in contact with an outside thermal reservoir, and a change in the system occurs slowly enough to allow the system to be continuously adjusted to the temperature of the reservoir through heat exchange (see quasi equilibrium).

Ideal Refrigeration Cycle P H Diagram 1в 2 Isobaric Cooling And Effect of cooling history in fe c system. eutectoid composition, co = 0.76 wt% c. begin at t > 727°c. rapidly cool to 625°c and hold isothermally. adapted from fig. 10.14,callister 7e. (fig. 10.14 adapted from. h. boyer (ed.) atlas of isothermal transformation and cooling transformation diagrams, american society for metals, 1997, p. 28.). An isothermal process is a type of thermodynamic process in which the temperature t of a system remains constant: Δt = 0. this typically occurs when a system is in contact with an outside thermal reservoir, and a change in the system occurs slowly enough to allow the system to be continuously adjusted to the temperature of the reservoir through heat exchange (see quasi equilibrium).
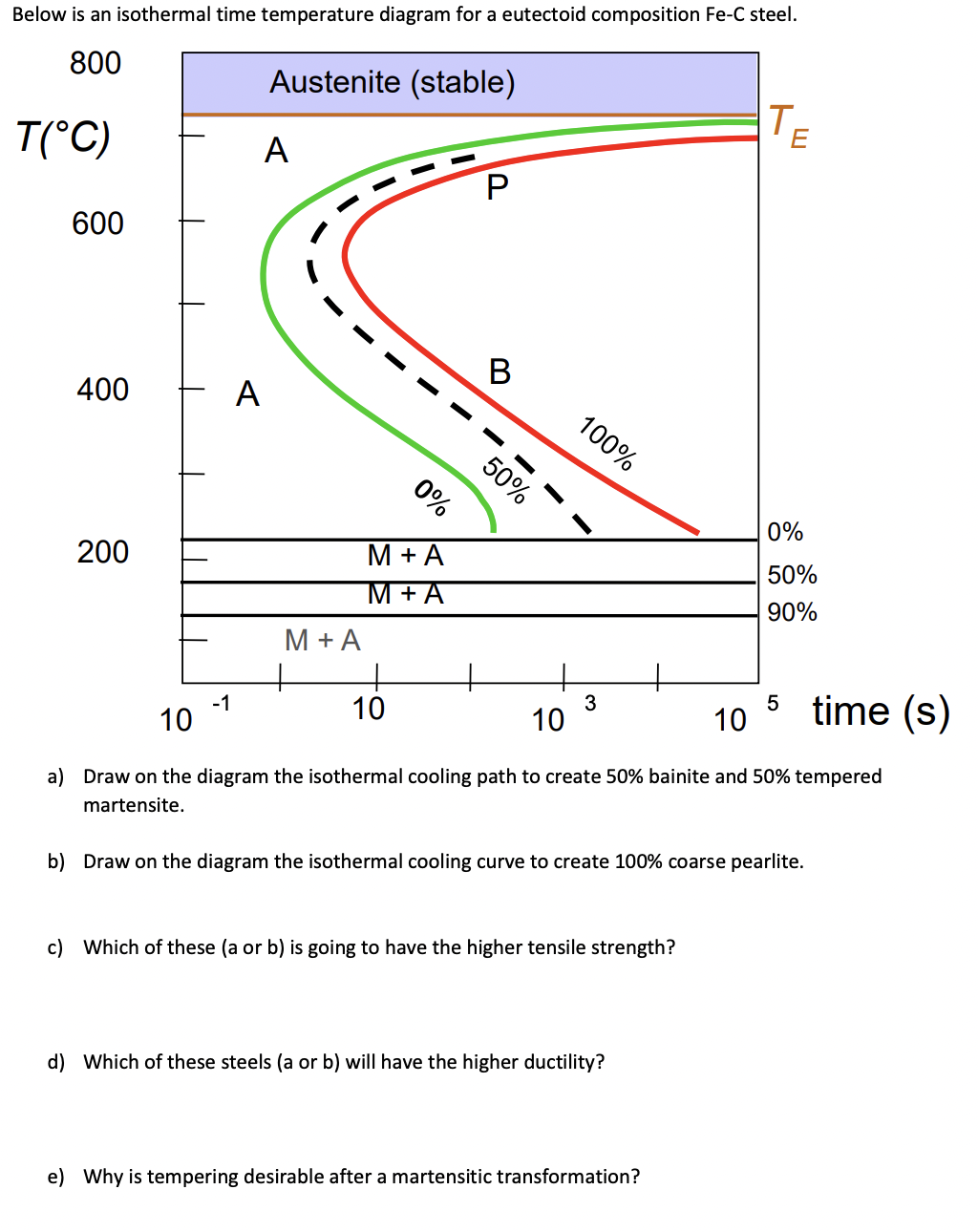
Solved A Draw On The Diagram The Isothermal Cooling Path To Chegg

Comments are closed.