Immunotherapy Drugs Approved For Bladder Cancer Nci
Atezolizumab For Bladder Cancer Nci The atezolizumab approval is an expansion of an earlier approval for bladder cancer. in 2016, fda approved the drug for patients with locally advanced or metastatic urothelial carcinoma that had gotten worse during or after treatment with cisplatin. however, genentech, the drug’s maker, reported on may 9 that, in a large clinical trial. This page lists cancer drugs approved by the food and drug administration (fda) for bladder cancer. the list includes generic names and brand names. the drug names link to nci’s cancer drug information summaries. there may be drugs used in bladder cancer that are not listed here.
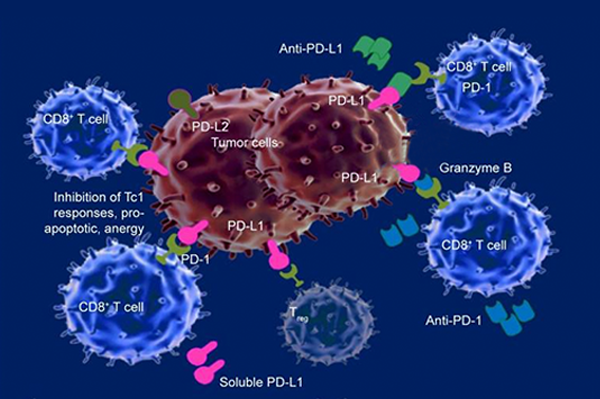
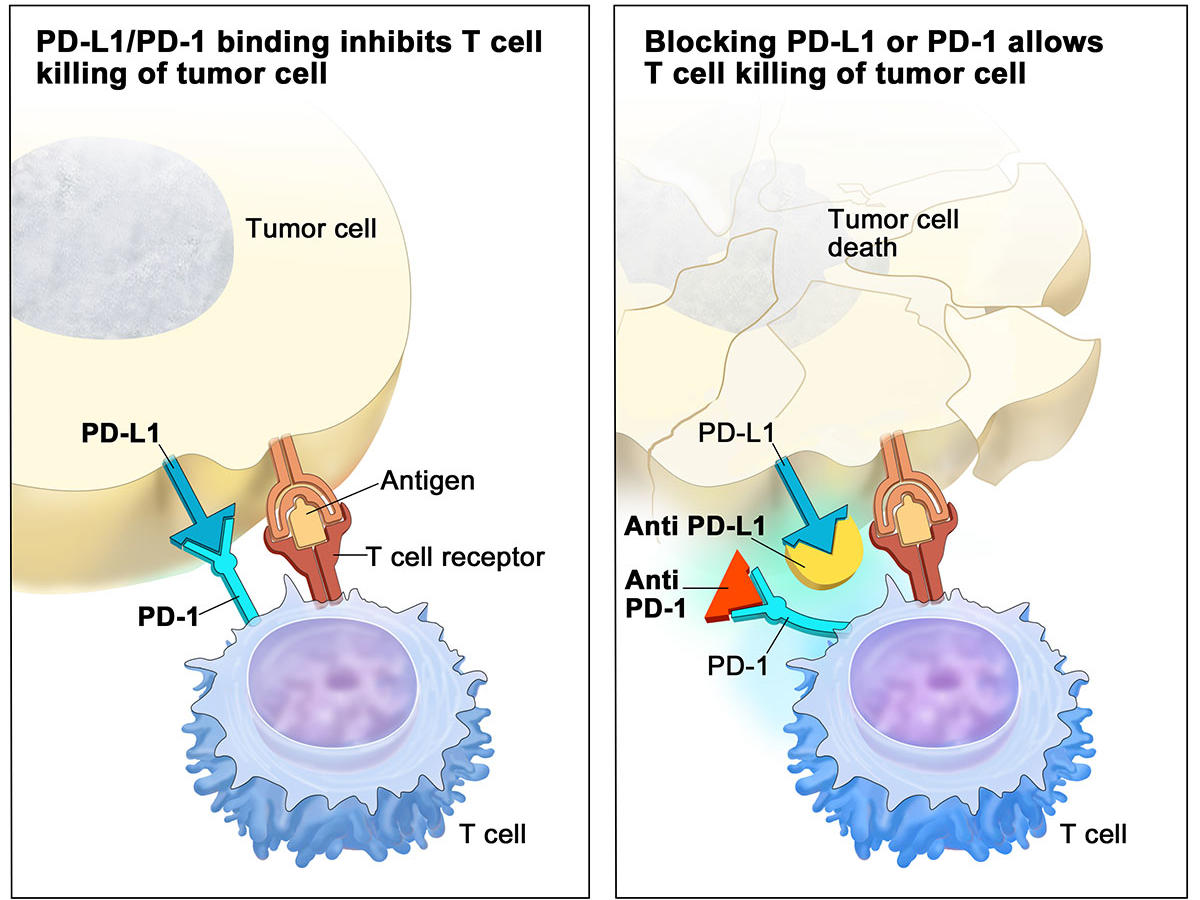
Checkpoint Inhibitor Use Changed For Bladder Cancer Nci Since 2016, the food and drug administration has approved five different immunotherapy drugs for the treatment of metastatic bladder cancer. these therapies all belong to a class of drugs called immune checkpoint inhibitors. checkpoint inhibitors bind to proteins that can keep cancer cells from being killed by the body’s immune cells. this. The fda has approved nogapendekin alfa inbakicept pmln, which stimulates immune cells to fight bladder cancer. the u.s. food and drug administration (fda) has approved nogapendekin alfa inbakicept pmln (anktiva) in combination with bacillus calmette guérin (bcg), for the treatment of certain adult patients with non muscle invasive bladder cancer (nmibc) that has not responded to bcg alone. This drug can be used along with the immunotherapy drug pembrolizumab (see above) in people with advanced bladder cancer. it can also be used by itself to treat people with advanced bladder cancer who: have already been treated with a platinum based chemo drug (such as cisplatin) and immunotherapy (specifically, a pd 1 or pd l1 inhibitor), or. As a result, these two drugs have been officially withdrawn from the second line treatment of bladder cancer . first line therapy pembrolizumab and atezolizumab were given accelerated approval for the first line treatment of cisplatin ineligible advanced or metastatic ubc, following keynote 052 ( 37 ) and imvigor210 ( 14 ) phase ii trials.
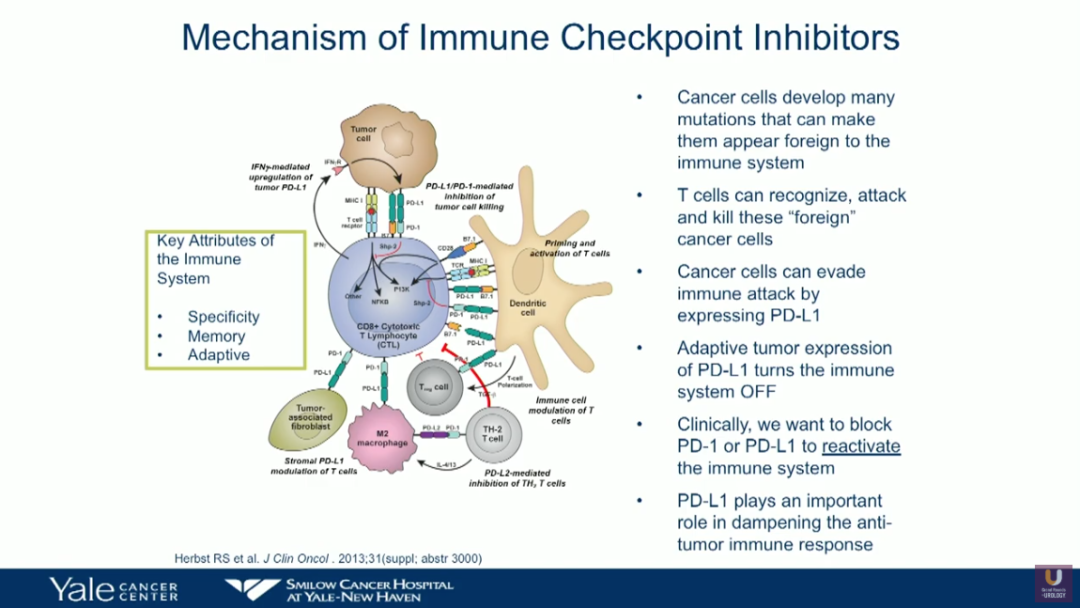
Bladder Cancer And Immunotherapy Daniel P Petrylak This drug can be used along with the immunotherapy drug pembrolizumab (see above) in people with advanced bladder cancer. it can also be used by itself to treat people with advanced bladder cancer who: have already been treated with a platinum based chemo drug (such as cisplatin) and immunotherapy (specifically, a pd 1 or pd l1 inhibitor), or. As a result, these two drugs have been officially withdrawn from the second line treatment of bladder cancer . first line therapy pembrolizumab and atezolizumab were given accelerated approval for the first line treatment of cisplatin ineligible advanced or metastatic ubc, following keynote 052 ( 37 ) and imvigor210 ( 14 ) phase ii trials. In 2023, the food and drug administration (fda) approved a two drug combination of enfortumab vedotin and pembrolizumab (ev pembro) to treat locally advanced or metastatic urothelial bladder cancer. the combination was previously only approved for the significant number of people who were ineligible for first line treatment with a type of. May 18, 2016. the u.s. food and drug administration today approved tecentriq (atezolizumab) to treat the most common type of bladder cancer, called urothelial carcinoma. this is the first product.

Bladder Cancer And Cancer Immunotherapy A New Path Forward In 2023, the food and drug administration (fda) approved a two drug combination of enfortumab vedotin and pembrolizumab (ev pembro) to treat locally advanced or metastatic urothelial bladder cancer. the combination was previously only approved for the significant number of people who were ineligible for first line treatment with a type of. May 18, 2016. the u.s. food and drug administration today approved tecentriq (atezolizumab) to treat the most common type of bladder cancer, called urothelial carcinoma. this is the first product.

Comments are closed.