Difference Between Physical Change And Chemical Change Comparison Between ођ

Difference Between Physical And Chemical Change Compare The Selected text level. matter is capable of undergoing changes, which are classified as either physical or chemical. physical changes in matter are often reversible: an ice cube can melt into liquid water, and then the liquid water can be frozen back into an ice cube. chemical changes, on the other hand, are not reversible: a log burned in a fire. A few examples of chemical change are digestion of food, burning of coal, rusting, etc. generally, physical changes do not involve the production of energy. chemical changes usually involve the production of energy (which can be in the form of heat, light, sound, etc.) in a physical change, no new substance is formed.
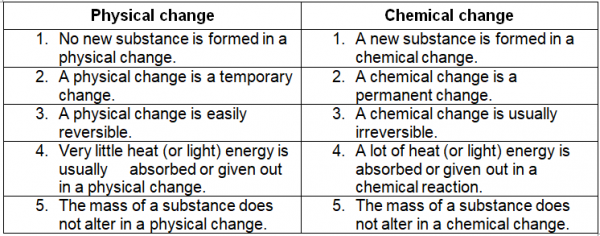
Difference Between Physical Change And Chemical Change Dunprime Chemical change vs. physical change. the difference between a physical reaction and a chemical reaction is composition. in a chemical reaction, there is a change in the composition of the substances in question; in a physical change there is a difference in the appearance, smell, or simple display of a sample of matter without a change in. Physical change refers to a change in which the molecules are rearranged but their internal composition remains same. chemical change is a process in which the substance transforms into a new substance, having different chemical composition. example. tearing of paper, melting freezing of water, cutting of trees, etc. Physical changes. a physical change is a change in matter that alters its form but not its chemical identity. the size or shape of matter often changes, but there is no chemical reaction. phase changes are physical changes. these include melting, boiling, vaporization, freezing, sublimation and deposition. breaking, crumpling, or molding matter. Matter is capable of undergoing changes, which are classified as either physical or chemical. physical changes in matter are often reversible. for example, an ice cube can melt into liquid water, and then the liquid water can be refrozen into an ice cube. a chemical change is very different. a burning log demonstrates this.

Comments are closed.