Decay Series Question 2

Decay Series Question 2 Youtube Nuclear physics. in nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. it is also known as a "radioactive cascade". the typical radioisotope does not decay directly to a stable state, but rather it decays to another radioisotope. Types of radioactive decay. ernest rutherford’s experiments involving the interaction of radiation with a magnetic or electric field (figure \(\pageindex{2}\)) helped him determine that one type of radiation consisted of positively charged and relatively massive \(α\) particles; a second type was made up of negatively charged and much less massive \(β\) particles; and a third was uncharged.

Solved 2 A Explicitly Explain The Following Decay Series Chegg The most common is the uranium 238 decay series, which produces lead 206 in a series of 14 sequential alpha and beta decay reactions (figure \(\pageindex{2}\)). although a radioactive decay series can be written for almost any isotope with z > 85, only two others occur naturally: the decay of uranium 235 to lead 207 (in 11 steps) and thorium. The eventual result will be an isotope of mass number 232 – 24 = 208 and a nuclear charge of 90 – 8 = 82. since element 82 is pb pb, we can write. th 90232 pb 82208 6 he24 4 e−1 0 th 90 232 pb 82 208 6 he 2 4 4 e − 1 0. 19.3: radioactive series. a radioactive series is a "decay chain" of radioactive decays of different. The rate for radioactive decay is: decay rate = λn with λ = the decay constant for the particular radioisotope. the decay constant, λ, which is the same as a rate constant discussed in the kinetics chapter. it is possible to express the decay constant in terms of the half life, t1 2: λ = ln 2 t1 2 = 0.693 t1 2 or t1 2 = ln 2 λ = 0.693 λ. Types of radioactive decay. ernest rutherford’s experiments involving the interaction of radiation with a magnetic or electric field (figure 21.6) helped him determine that one type of radiation consisted of positively charged and relatively massive α particles; a second type was made up of negatively charged and much less massive β particles; and a third was uncharged electromagnetic.
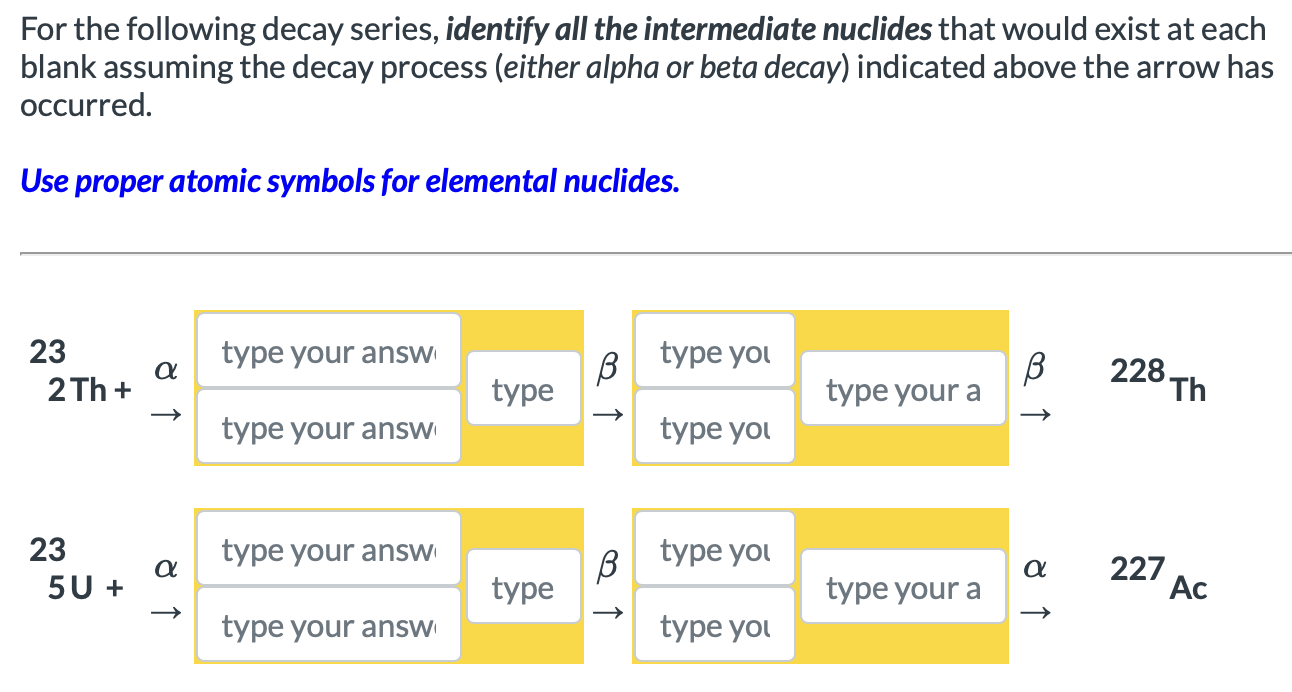
Solved For The Following Decay Series Identify All The Chegg The rate for radioactive decay is: decay rate = λn with λ = the decay constant for the particular radioisotope. the decay constant, λ, which is the same as a rate constant discussed in the kinetics chapter. it is possible to express the decay constant in terms of the half life, t1 2: λ = ln 2 t1 2 = 0.693 t1 2 or t1 2 = ln 2 λ = 0.693 λ. Types of radioactive decay. ernest rutherford’s experiments involving the interaction of radiation with a magnetic or electric field (figure 21.6) helped him determine that one type of radiation consisted of positively charged and relatively massive α particles; a second type was made up of negatively charged and much less massive β particles; and a third was uncharged electromagnetic. There are three major types of nuclear decay, called alpha (α) beta (β) and gamma (γ). the α decay equation is a zxn →a − 4 z − 2yn − 2 4 2he2. nuclear decay releases an amount of energy e related to the mass destroyed Δm by e = (Δm)c2. there are three forms of beta decay. In this case, we use the term decay series or decay chain. now, the mass number decreases by 4 or remains constant at any decay. now, the mass number decreases by 4 or remains constant at any decay. if we divide the mass numbers for the nuclides of the same decay series by 4, the remainder will always be the same (0, 1, 2 or 3).

Oneclass 2 Th 232 Decay Series Complete The Following Decay Series There are three major types of nuclear decay, called alpha (α) beta (β) and gamma (γ). the α decay equation is a zxn →a − 4 z − 2yn − 2 4 2he2. nuclear decay releases an amount of energy e related to the mass destroyed Δm by e = (Δm)c2. there are three forms of beta decay. In this case, we use the term decay series or decay chain. now, the mass number decreases by 4 or remains constant at any decay. now, the mass number decreases by 4 or remains constant at any decay. if we divide the mass numbers for the nuclides of the same decay series by 4, the remainder will always be the same (0, 1, 2 or 3).

Answered 2 Fill In The Blanks In The Followingвђ Bartleby

Comments are closed.