Chemical Change Physical Properties With Bread Vs Toast Matter

Chemical Change Physical Properties With Bread Vs Toast Matter Put back in bowl and let rise in a warm place. when doubled in size (1 hour or so), punch down and form into two loaves. place in well greased bread pans. cover with cloth and let rise for 45 minutes or so. bake at 175 °c (350 °f) for 45 minutes in a regular oven, a bit less in a convection oven. Many people wonder if toasting bread is a physical change. the answer is yes, it is. when bread is toasted, it undergoes various chemical and physical changes that affect its texture, aroma, taste, and nutritional value. toasting bread is important because it improves the flavor profile in several ways.
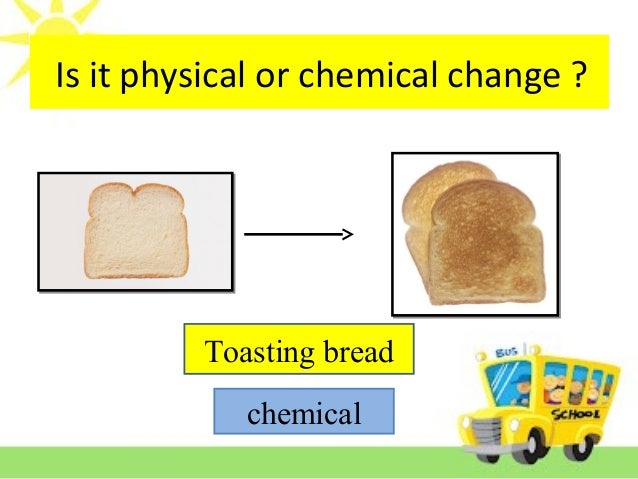
Is Baking Bread A Chemical Or Physical Change Bread Poster The difference between a physical change and a chemical change (or reaction) is composition. in a chemical change, there is a change in the composition of the substances in question; in a physical change there is a difference in the appearance, smell, or simple display of a sample of matter without a change in composition. Selected text level. matter is capable of undergoing changes, which are classified as either physical or chemical. physical changes in matter are often reversible: an ice cube can melt into liquid water, and then the liquid water can be frozen back into an ice cube. chemical changes, on the other hand, are not reversible: a log burned in a fire. Color changes indicate chemical change. the following reaction occurs: fe o2 → fe2o3 fe o 2 → fe 2 o 3. physical: because none of the properties changed, this is a physical change. the green mixture is still green and the colorless solution is still colorless. they have just been spread together. Physical changes. a physical change is a change in matter that alters its form but not its chemical identity. the size or shape of matter often changes, but there is no chemical reaction. phase changes are physical changes. these include melting, boiling, vaporization, freezing, sublimation and deposition. breaking, crumpling, or molding matter.
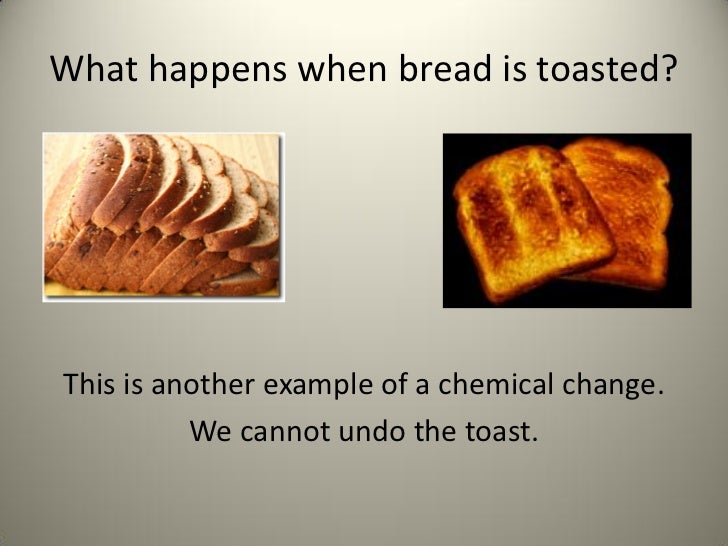
Is Baking Bread A Chemical Or Physical Change Bread Poster Color changes indicate chemical change. the following reaction occurs: fe o2 → fe2o3 fe o 2 → fe 2 o 3. physical: because none of the properties changed, this is a physical change. the green mixture is still green and the colorless solution is still colorless. they have just been spread together. Physical changes. a physical change is a change in matter that alters its form but not its chemical identity. the size or shape of matter often changes, but there is no chemical reaction. phase changes are physical changes. these include melting, boiling, vaporization, freezing, sublimation and deposition. breaking, crumpling, or molding matter. Bread must be baked. in bread, temperatures from 140° to 165° c trigger the maillard reaction. in this process, sugars and the amino acids in protein undergo multiple chemical reactions to form. Grouped together, they form molecules. matter can go through changes. some changes to matter are physical. other changes are chemical. the two kinds of changes are very different from each other. physical changes can often be reversed. for example, think of an ice cube in a hot room. the ice will melt and become water.

Is Baking Bread A Chemical Or Physical Change Bread Poster Bread must be baked. in bread, temperatures from 140° to 165° c trigger the maillard reaction. in this process, sugars and the amino acids in protein undergo multiple chemical reactions to form. Grouped together, they form molecules. matter can go through changes. some changes to matter are physical. other changes are chemical. the two kinds of changes are very different from each other. physical changes can often be reversed. for example, think of an ice cube in a hot room. the ice will melt and become water.

Comments are closed.