Carbon Disulfide Cs2 Boils At 46 30 Ac And Has A Density Of 1 261 Gml Part A When 0 240 Mol Of A N
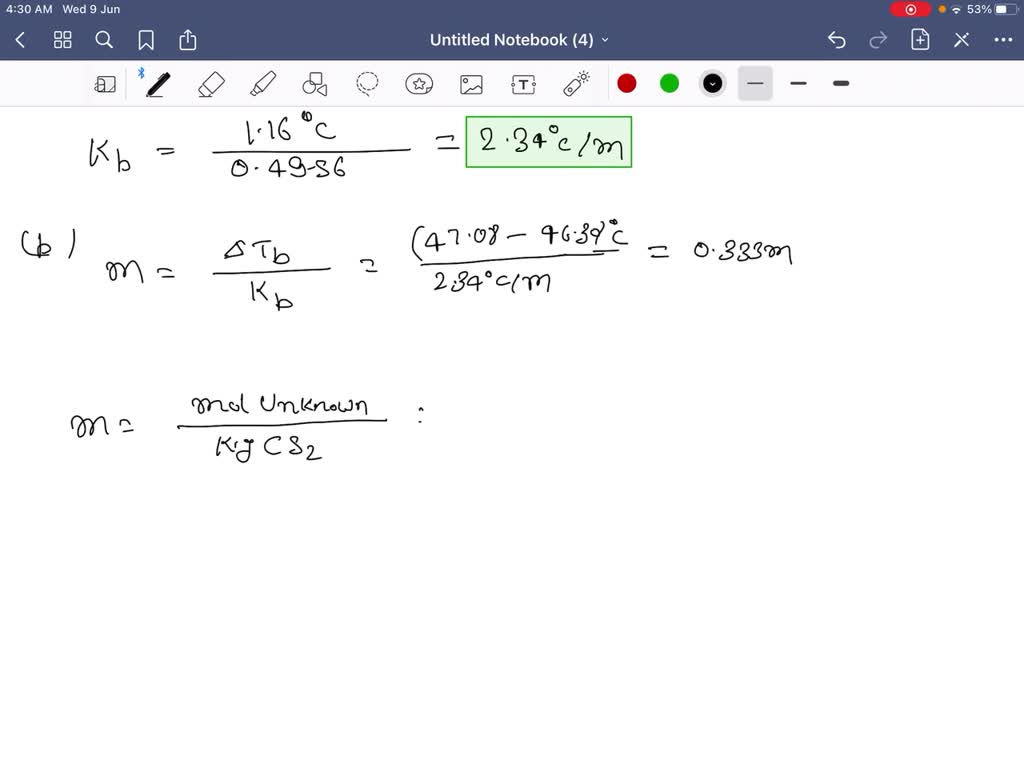
Solved юааcarbonюаб юааdisulfideюаб юааcs2юаб юааboilsюаб юааat 46юаб юаа30юаб тиш C юааand Hasюаб ю Carbon disulfide (cs2) boils at 46.30 .c and has a density of 1.261 g ml. part a: when 0.240 mol of a nondissociating solute is dissolved in 410.0 ml of cs2, the solution boils at 47.39 ac. what is the molal boiling point elevation constant for cs2? part b: when 5.38 g of a nondissociating unknown is dissolved in 50.0 ml of cs2, the solution. Carbon disulfide (cs2) boils at 46.30 °c and has a density of 1.261 gml. part a when 0.240 mol of a nondissociating solute is dissolved in 410.0 ml of cs2,.

Solved Carbon Disulfide Cs2 Boils At 46 30 г в C And Has о Video answer: the question is about the boiling point of the carbon disulfide, which is 46.30 degree celsius. the first question we have to answer is what is the boiling point elevation constant. Therefore, delta t equals to 47.46 degree celsius. minus 46.30 degree celsius that is equal to 1.16 degree. elzemorality m equals to a weight of solvent in k. that is c s. 2 pot now put the value 0.250 moles of solute divided by 400 ml s to multiply by so we need to convert 400 ml of c h, 2 into kg, so multiplies density that is 1.261 gram. Theodore l. brown 14th edition. chapter 13. sections. step by step solved, expert educator: carbon disulfide (cs2) ( c s 2) boils at. transcript. video answer: to calculate the mass of c is 2 mass of mass. if density is gram per mole into volume, which is so here, density is 1.126 gram per mole and 400 mon, which is 504.4 grams. Strong field vs weak field ligands. 6m. magnetic properties of complex ions: octahedral complexes. 11m. carbon disulfide 1cs22 boils at 46.30 °c and has a density of 1.261 g>ml. (a) when 0.250 mol of a nondissociating solute is dissolved in 400.0 ml of cs2, the solution boils at 47.46 °c.

Comments are closed.