Calculate The Emf Of The Cell Zn Zn2 0 1 M Cd2 0 01 M Cd At
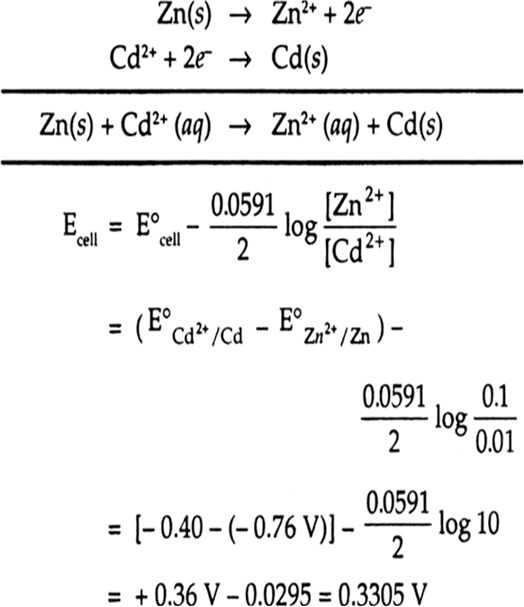
Calculate The Emf Of The Cell Zn Zn2 0 1 M Cd2 0о Click here:point up 2:to get an answer to your question :writing hand:calculate the emf of the cell zn zn2 0001 m cu2 01 m. The emf of a cell : z n 2 h → z n 2 (0.1 m) h 2 (1 a t m) is 0.28 v at 25 ∘ c. calculate ph of the solution at the hydrogen gas electrode. calculate ph of the solution at the hydrogen gas electrode.
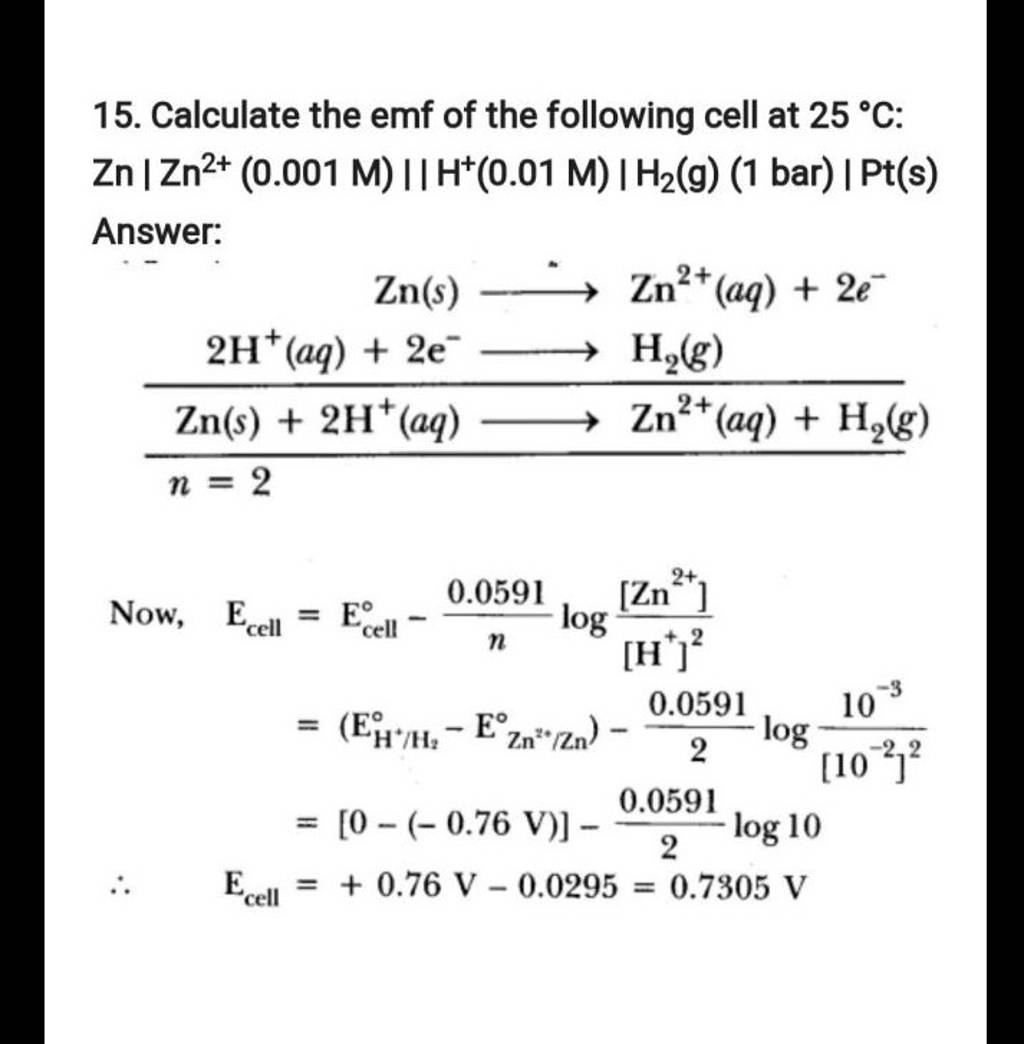
15 юааcalculateюаб юааthe Emfюаб Of The Following юааcellюаб At 25тишc юааznюабтигтиг юааzn2юаб юаа0 The emf of the cell, z n | z n 2 (0.01 m) | | f e 2 (0.001 m) | f e at 298 k is 0.2905 v then the value of equilibrium constant for the cell reaction is: view solution. The following curve is obtained when molar conductivity λ m (y axis) is plotted against the square root of concentration c 1 2 (x axis) for two electrolytes a and b. (a) what can you about the nature of the two electrolytes a and b. The emf of the cell with the cell reaction given below is 0.28 v at 25 o c. z n ( s ) 2 h ( a q ) → z n 2 ( a q , 0.1 m ) h 2 ( g , 1 a t m calculate the p h of the hydrogen electrode. Calculate the emf of the following cell at 25 °c: zn | zn2 (0.001 m) || h (0.01 m) | h2(g) (1 bar) | pt(s)watch the full video at: numerade.

Solved A Calculate The Emf Of The Following Cell Zn Zn2 Chegg The emf of the cell with the cell reaction given below is 0.28 v at 25 o c. z n ( s ) 2 h ( a q ) → z n 2 ( a q , 0.1 m ) h 2 ( g , 1 a t m calculate the p h of the hydrogen electrode. Calculate the emf of the following cell at 25 °c: zn | zn2 (0.001 m) || h (0.01 m) | h2(g) (1 bar) | pt(s)watch the full video at: numerade. A) calculate the emf of the cell represented below: zn zn2 (c = 0.1m) | cu 2 (c = 1m) cu at 25°c given : eºcu = 0.34 v and en = 0.76 v open in app solution. A) calculate the emf of the following cell zn zn2 (0.1m) ii ag (0.2m) ag; given e°zn2 zn = 0.76 v and eºagt ag = 0.8 v 2 b) a cu rod is placed in 0.01 m cuso4 solution at 298k. calculate the potential of the electrode assuming that salt is dissociated to 90 % at this dilution. given e° for cu2 cu = 0.34 v.
Solved Calculate The Emf Of A Cell Using The Nernst Equation Zn Zn A) calculate the emf of the cell represented below: zn zn2 (c = 0.1m) | cu 2 (c = 1m) cu at 25°c given : eºcu = 0.34 v and en = 0.76 v open in app solution. A) calculate the emf of the following cell zn zn2 (0.1m) ii ag (0.2m) ag; given e°zn2 zn = 0.76 v and eºagt ag = 0.8 v 2 b) a cu rod is placed in 0.01 m cuso4 solution at 298k. calculate the potential of the electrode assuming that salt is dissociated to 90 % at this dilution. given e° for cu2 cu = 0.34 v.

17 The Emf Of A Cell Zn Zn2 0 01 M Physical Chemistry
Comments are closed.