Are Catalyst Consumed In A Reaction

What Is A Catalyst Understand Catalysis In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalysis is the process of speeding up a reaction using a catalyst. the word “catalyst” comes from the greek word kataluein, which means to loosen or untie. british chemistry elizabeth fulhame first described the. 14.7: catalysis.

Catalysts Of Chemical Reactions At Kimberly Ray Blog Catalyst | examples, definition, & facts. What is a catalyst? a catalyst is a substance that is used to speed up a chemical reaction but it is not consumed by the reaction. what is catalysis? catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. a very small amount of catalyst is required to alter the reaction. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. this process is called catalysis. a catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. the only difference between a catalyzed reaction and an. Catalysis | chemistry, classification, & chemical reactions.
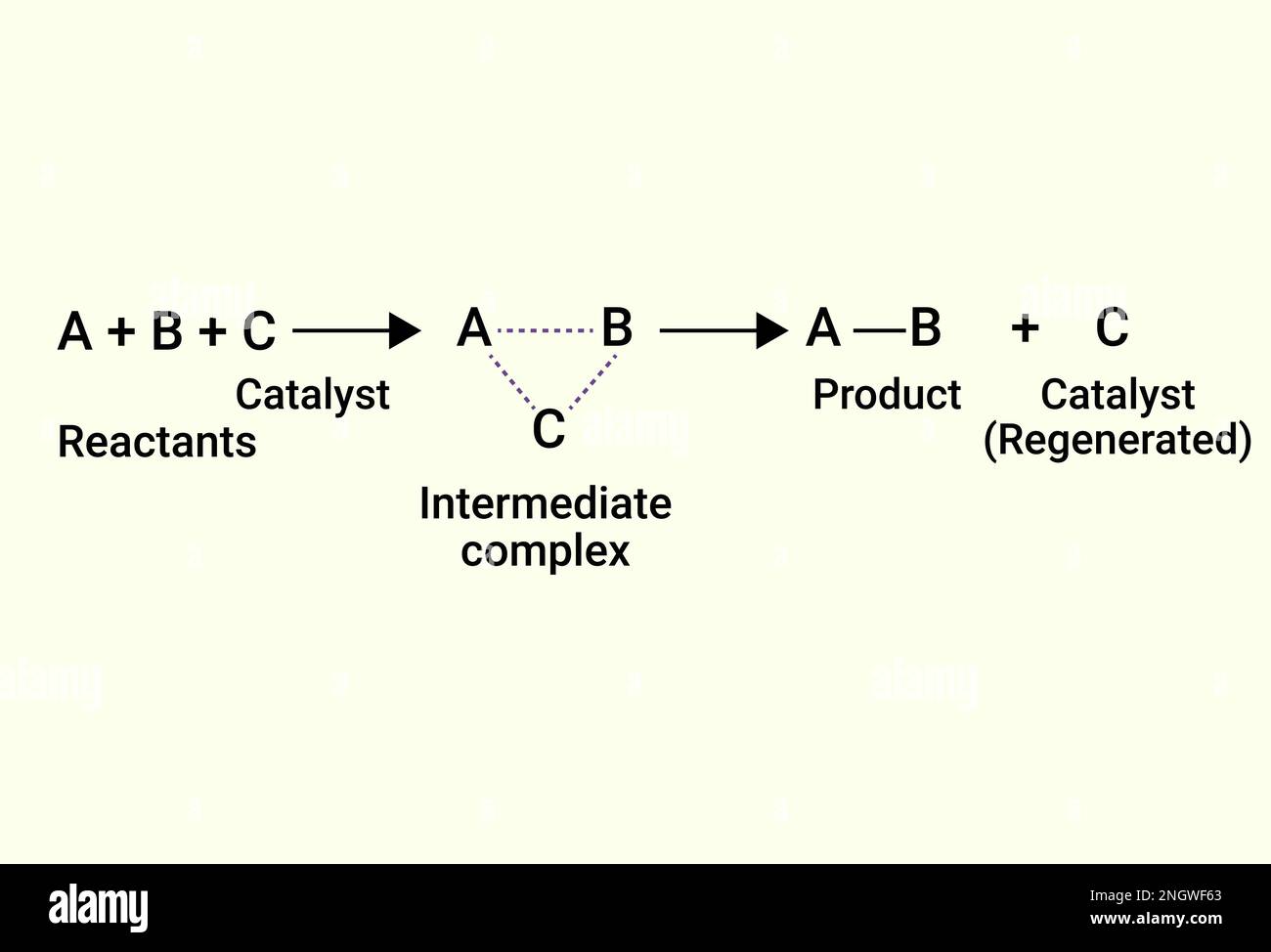
Chemical Reactions Of Catalyst And Product Stock Vector Image Art Alamy A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. this process is called catalysis. a catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. the only difference between a catalyzed reaction and an. Catalysis | chemistry, classification, & chemical reactions. A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. catalysis is the process of adding a catalyst to facilitate a reaction. during a chemical reaction, the bonds between the atoms in molecules are broken, rearranged, and. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can increase the reaction rate without being consumed in the reaction. the concepts introduced in the previous section on reaction mechanisms provide the basis for understanding how catalysts are able to.
Determining How A Catalyst Can Be Consumed Regenerated In A Reaction A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction. catalysis is the process of adding a catalyst to facilitate a reaction. during a chemical reaction, the bonds between the atoms in molecules are broken, rearranged, and. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can increase the reaction rate without being consumed in the reaction. the concepts introduced in the previous section on reaction mechanisms provide the basis for understanding how catalysts are able to.

Comments are closed.