16 43a Calculate The Equilibrium Constant For O2g 2f2g → 2f2og T 100 C
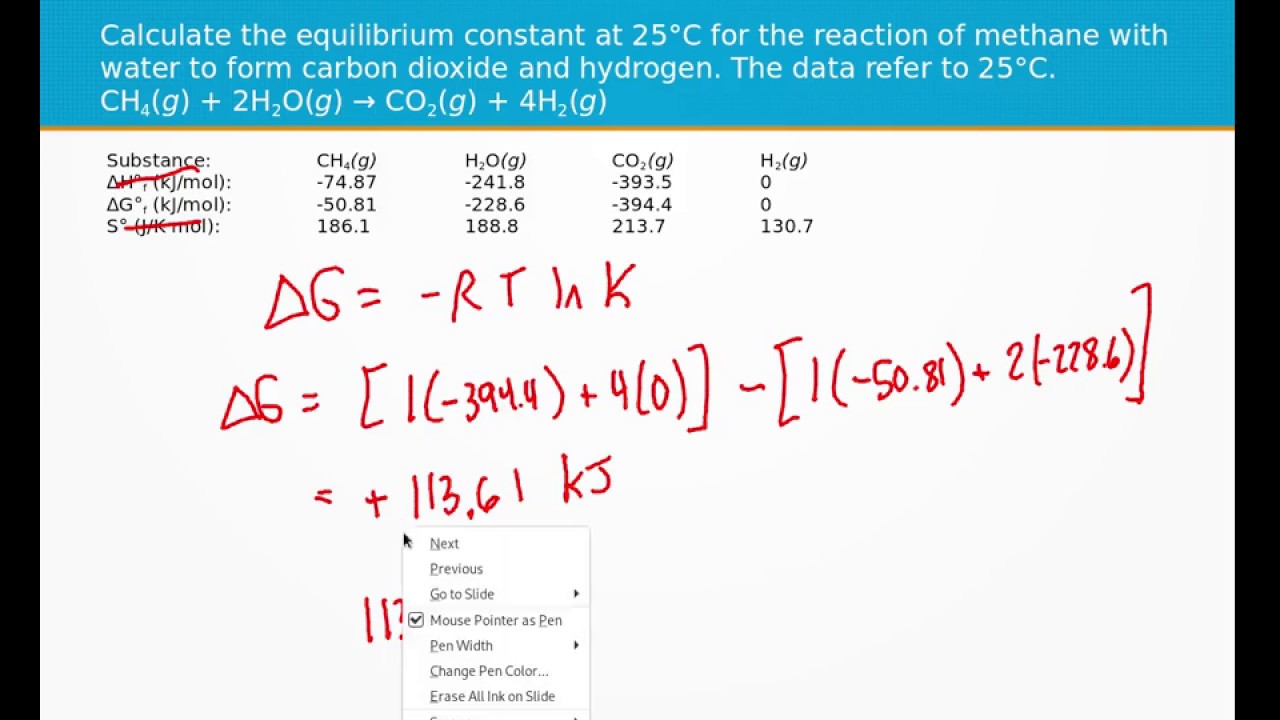
Calculate The Equilibrium Constant Youtube Calculate the equilibrium constant at the temperature given.o2(g) 2f2(g) → 2f2o(g) (t = 100 °c)openstax™ is a registered trademark, which was not involved. The equilibrium constant of a reaction relates to all of the species present in the reaction. however, in this calculator, we assume that there is a maximum of two main reactants and two main products. for the hypothetical reaction: a[a] b[b] ⇌ c[c] d[d] the equilibrium constant equation has the following formula: k = ([c] c × [d] d) ([b.

Solved Calculate The Equilibrium Constant At The Given Temperature A Question: 43. calculate the equilibrium constant at the temperature given. (a) o2(g) 2f2(g) → 2f2o(g) (t = 100 °c) (b) 12(s) br2(l) — 21br(g) (t = 0.0 °c. Kc is the equilibrium constant that is defined based on the molar concentrations of reactants and products. it is calculated for a generic reaction of the form aa bb ⇌ cc dd, where a, b, c, and d are the chemical species involved and a, b, c, and d are their respective stoichiometric coefficients. the expression for kc is given by: k c. 43. calculate the equilibrium constant at the temperature given. (a) o2(g) 2f2(g) → 2f20(g) (b) 12(s) br2(l) 21br(g) (c) 2lioh(s) co2(g) → li 2 co3(s) h2o. Substitute into the equilibrium expression and solve for k; example: initially, a mixture of 0.100 m no, 0.050 m h 2, 0.100 m h 2 o was allowed to reach equilibrium (initially there was no n 2). at equilibrium the concentration of no was found to be 0.062 m. determine the value of the equilibrium constant, k c, for the reaction:.

Comments are closed.